
For the hydrogen molecule, the H-H bond strength is equal to about 435 kJ/mol.Įvery covalent bond in a given molecule has a characteristic length and strength.
Likewise, the difference in potential energy between the lowest energy state (at the optimal internuclear distance) and the state where the two atoms are completely separated is called the bond dissociation energy, or, more simply, bond strength.

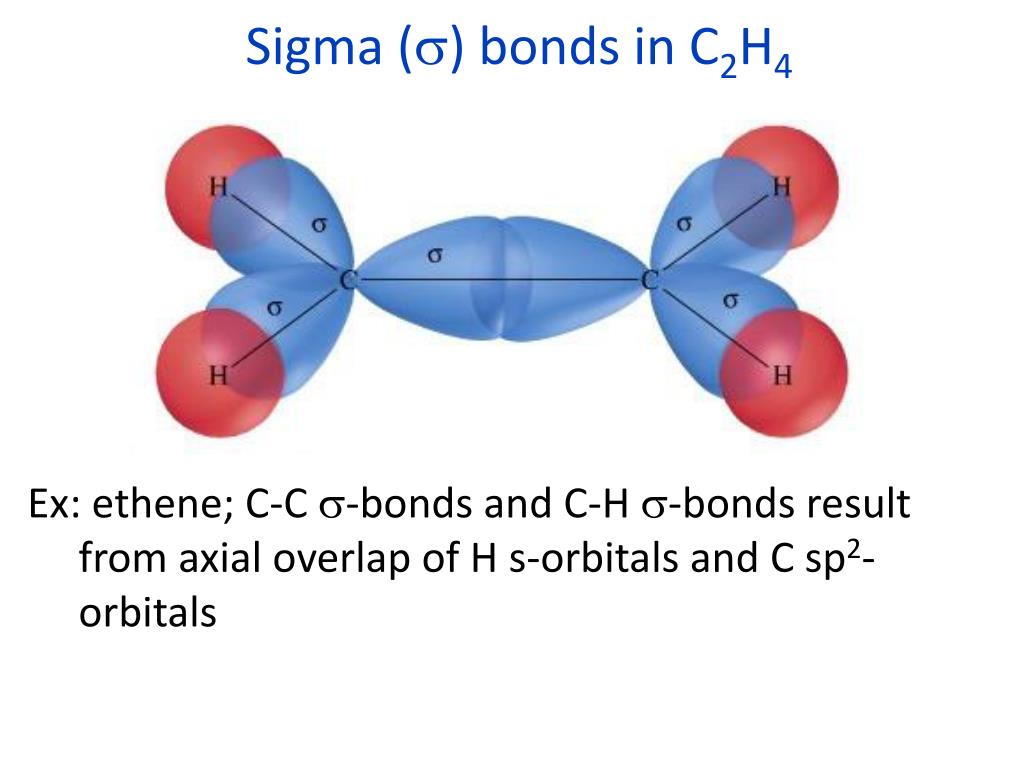
For the H 2 molecule, the distance is 74 pm (picometers, 10 -12 meters). This optimal internuclear distance is the bond length. There is a defined optimal distance between the nuclei in which the potential energy is at a minimum, meaning that the combined attractive and repulsive forces add up to the greatest overall attractive force. When the two nuclei are ‘too close’, we have an unstable, high-energy situation. This lowers the potential energy of the system, as new, attractive positive-negative electrostatic interactions become possible between the nucleus of one atom and the electron of the second.īut something else is happening at the same time: as the atoms get closer, the repulsive positive-positive interaction between the two nuclei also begins to increase.Īt first this repulsion is more than offset by the attraction between nuclei and electrons, but at a certain point, as the nuclei get even closer, the repulsive forces begin to overcome the attractive forces, and the potential energy of the system rises quickly. As they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. How far apart are the two nuclei? If they are too far apart, their respective 1 s orbitals cannot overlap, and thus no covalent bond can form - they are still just two separate hydrogen atoms. These two electrons are now attracted to the positive charge of both of the hydrogen nuclei, with the result that they serve as a sort of ‘chemical glue’ holding the two nuclei together. When we say that the two hydrogen nuclei share their electrons to form a covalent bond, what we mean in valence bond theory terms is that the two spherical 1 s orbitals (the grey spheres in the figure below) overlap, and contain two electrons with opposite spin. The simplest case to consider is the hydrogen molecule, H 2. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the sigma bond framework of the ethylene molecule.\) The C-C sigma bond in ethylene is formed by the overlap of an sp 2 hybrid orbital from each carbon. Consequently, consistent with the observations, the four carbon-hydrogen bonds in ethylene are identical. Thus, overlap two sp 2-hybridized orbitals with the 1s orbitals of two hydrogen atoms for the C-H sigma bonds in ethylene (sp 2(C)-1s(H). In the ethylene molecule, each carbon atom is bonded to two hydrogen atoms. The unhybridized 2 p z orbital is perpendicular to the plane of the trigonal planar sp 2 hybrid orbtals.
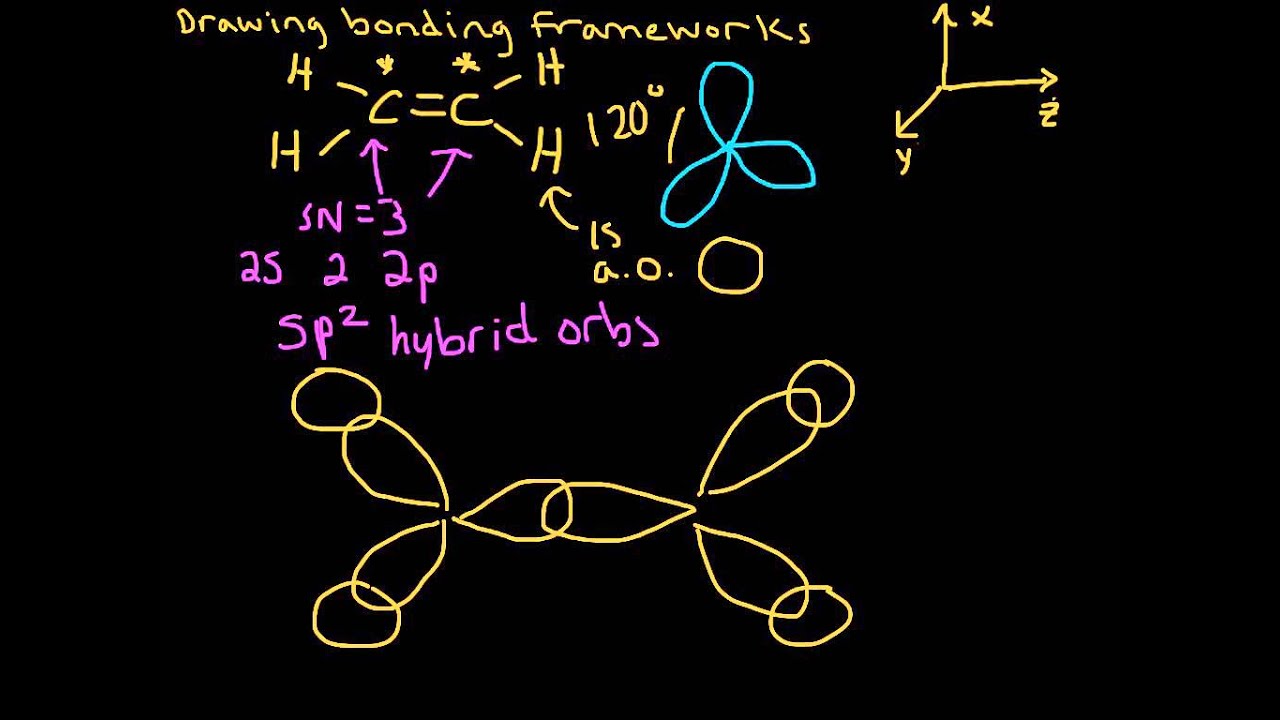
Atoms surrounded by three electron groups can be said to have a trigonal planar geometry and sp 2 hybridization. Again, geometry and hybrization can be tied together. Each orbital lobe is pointing to the three corners of an equilateral triangle, with angles of 120° between them. To minimize the repulsion between electrons, the three sp 2-hybridized orbitals are arranged with a trigonal planar geometry. The shape of the sp 2-hybridized orbital has be mathematically shown to to be roughly the same as that of the sp 3-hybridized orbital. However, the unpaired electrons are contained in two different types of orbitals so it is to be expected that two different types of bonds will form. Each carbon in ethene is said to be a “sp 2-hybridized carbon.” The electron configuration of the sp 2 hybridized carbon shows that there are four unpaired electrons to form bonds.
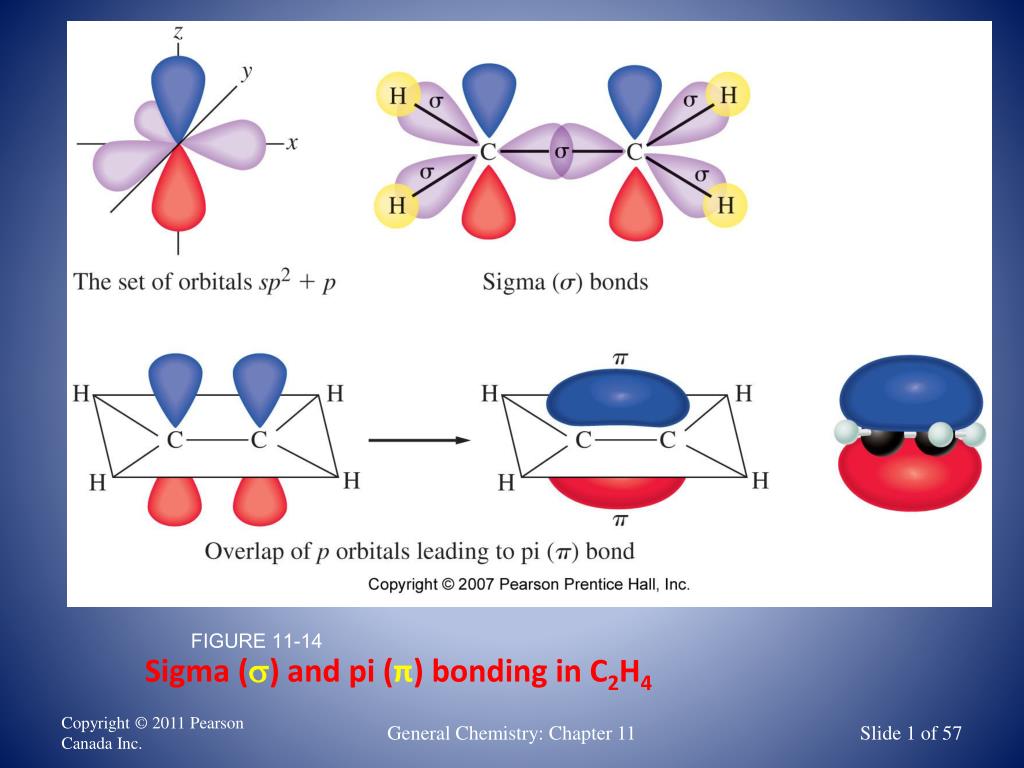
Three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the remaining electron goes into the unhybridized p z orbital. Three atomic orbitals on each carbon – the 2 s, 2 p x and 2 p y – combine to form three sp 2 hybrids, leaving the 2 p z orbital unhybridized. The sigma bonds formed in ethene is by the participation of a different kind of hybrid orbital.


 0 kommentar(er)
0 kommentar(er)
